

Organic reactions are the chemical reactions involving the organic compounds. What are the 6 types of functional groups Hydroxyl, sulfhydryl, carbonyl, carboxyl, amino and phosphate groups. Both balls are unchanged afterwards but are moving in different directions at new velocities. functional group, any of numerous combinations of atoms that form parts of chemical molecules, that undergo characteristic reactions themselves, and that in many cases influence the reactivity of the remainder of each molecule. This is rather like a collision in snooker or pool.
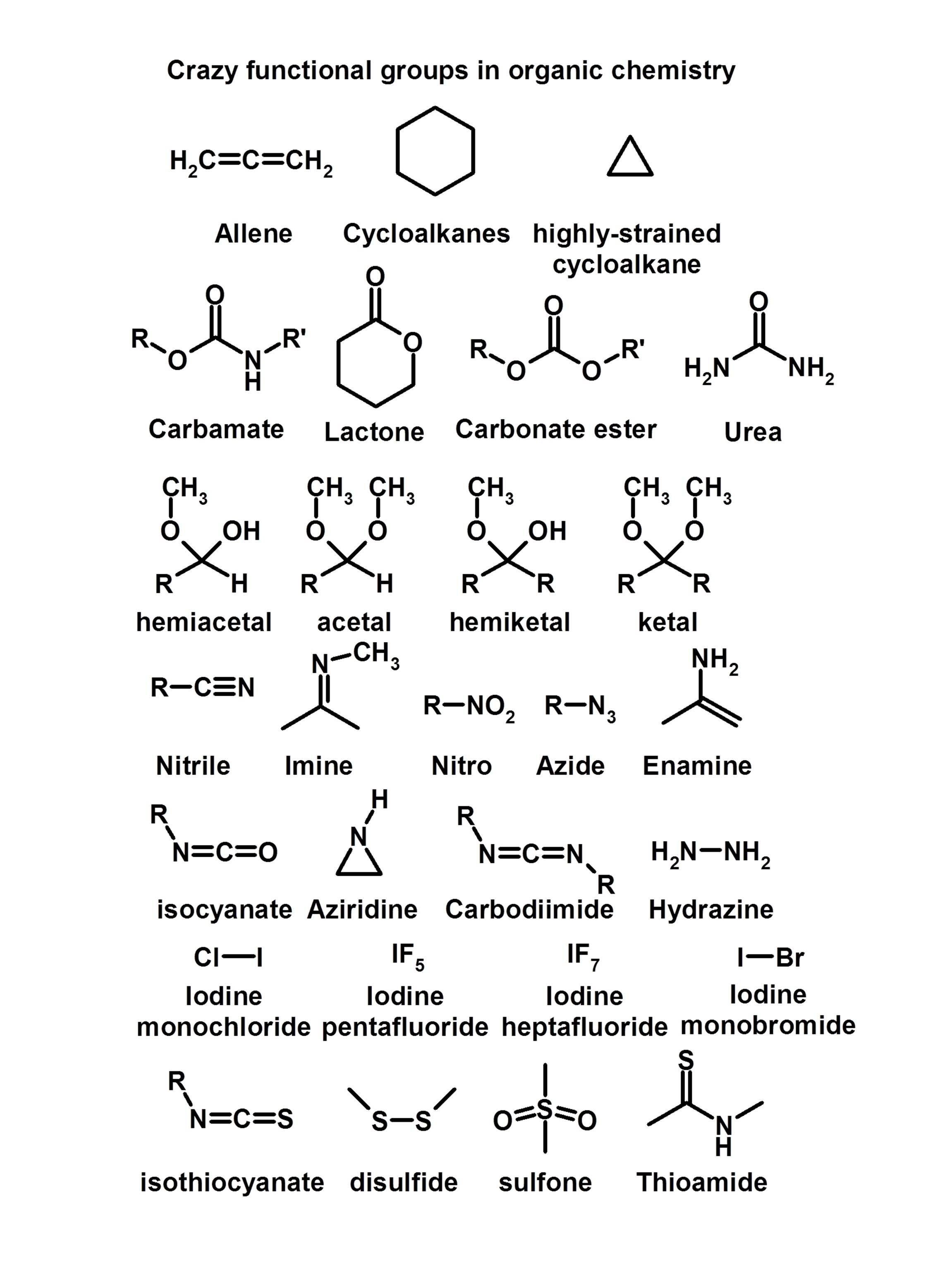
The functional group is an atom, or a group of atoms that has. If two molecules lack the required activation energy, they will simply collide, each bouncing off the electrons on the surface of the other and exchanging energy as they do so, but remain chemically unchanged. , for example a chain of sp3 hybridized carbon atoms, and one or several functional groups. Reaction will occur only if the molecules are given enough energy (the activation energy for the reaction) for the molecules to pass the repulsion and get close enough to each other. Charge–charge repulsion between these electrons ensures that all molecules repel each other. These moieties (the part of the molecule which can be found in many other molecules as well) are responsible for the chemical reactions that the molecule they are attached to participate in. The creation of new molecules is the special concern of chemistry and an interest in the mechanism of chemical reactions is the special concern of organic chemistry.Īll organic molecules have an outer layer of many electrons, which occupy filled orbitals, bonding and nonbonding. Functional Groups, in the field of organic chemistry, are the substituent atoms or groups of atoms that are attached to specific molecules. The first is static and the second dynamic. The second is the description of the reaction mechanism in terms of curly arrows and that is what we are about to start. One, is the structure and representation of molecules.
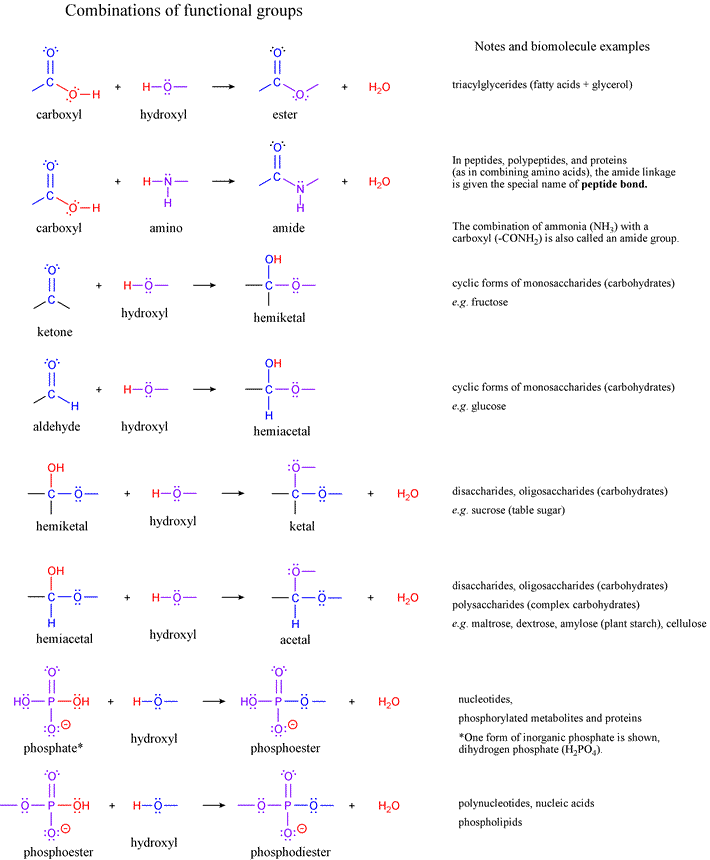
To understand organic chemistry you must be familiar with two languages. Nonpolar amino acids are hydrophobic which means they do not tend to move or combine with other aqueous compounds. Amino Acids with Nonpolar side chains These are amino acids or organic compounds that have no charge on the R group. Yet when we add chemical reagents, say, HCl to water, sodium cyanide (NaCN) to acetone, or sodium hydroxide to methyl iodide, chemical reactions occur. There are three (3) Major types of Functional Groups based on the R group of the said amino acid. Bottles of water, or acetone (propanone, Me 2C=O), or methyl iodide (iodomethane CH 3I) can be stored for years without any change in the chemical composition of the molecules inside. Most molecules are at peace with themselves.


 0 kommentar(er)
0 kommentar(er)
